Detecting brain diseases early
Until recently, a diagnosis of dementia might help explain cognitive decline, but there were no available treatments. In the last several years, the first drugs to slow the progression of Alzheimer’s have reached the market. This special Research in Context feature explores approaches to the diagnosis of Alzheimer’s and other dementias and the development of strategies to try to prevent their progression.
—by Sharon Reynolds
Maryland, USA: Diseases that degrade the mind strike at the very heart of our humanity. Unfortunately, such diseases aren’t rare. Strokes, infections, and chronic conditions like diabetes can all cause lasting damage to the brain. And more than 6 million Americans are living with Alzheimer’s disease and other dementias—a number that is expected to rise over the coming decades.
Dementia is a loss of thinking, remembering, and reasoning skills that affects everyday life. The risk for developing dementia increases sharply with age, but dementia is not part of the healthy aging process.
“When [older] people start to have memory problems, cognitive problems, it’s due to a disease. It’s not a normal consequence of aging,” explains Dr. Michael Weiner, a dementia researcher at the University of California, San Francisco.
While there are several different forms of dementia, Alzheimer’s disease is the most common. Until recently, a diagnosis of dementia might help explain cognitive decline, but there were no treatments available to change the course of the disease. However, the last several years have brought a major change, as the first drugs that can slow the progression of Alzheimer’s have reached the market.
NIH-funded researchers are now drawing on decades of advances in detecting the changes that lead to Alzheimer’s and other dementias to develop strategies to try to prevent or slow their progression.
“There’s really consistent evidence that the process of Alzheimer’s disease in the brain begins at least a decade before symptoms appear—maybe even two decades,” explains Dr. Reisa Sperling, an Alzheimer’s disease researcher at Harvard Medical School. The same appears to be true for many other dementias. “But that’s a glass-half-full situation, because it means we can potentially detect the disease before there is irreversible damage.”
Seeing damage in the brain
Some types of damage to the brain, like from a stroke, are relatively easy to pick out with a CT scan in the emergency department. But the brain changes associated with dementia start at the molecular level and get worse slowly. In the past, this made them difficult to detect.
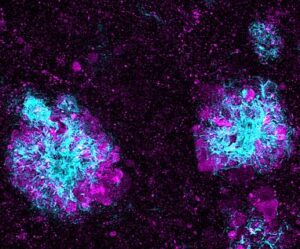
For example, until fairly recently, Alzheimer’s disease could only be confirmed after death. Characteristic damage to the brain could be seen during an autopsy. This damage includes abnormal clumps, called plaques, of a protein called amyloid-beta. It also involves bundles of fibers, or tangles, formed by another protein called tau.
Together, these harmful substances are thought to gum up the activity of neurons—the brain cells that convey chemical and electrical messages to create thoughts. This is associated with a progressive worsening of the cognitive symptoms of Alzheimer’s disease.
“Traditionally, the diagnosis of Alzheimer’s disease in patients was based on the clinical symptoms of dementia,” explains Dr. Eliezer Masliah, an aging expert at NIH.
In the early 2000s, biomarkers to diagnose Alzheimer’s disease in living people first became available for research. Broadly, a biomarker is any measurable characteristic that can reliably help diagnose disease. Biomarkers may include molecules found in blood tests, results from imaging exams, or other characteristics.
For Alzheimer’s disease, a breakthrough came with positron emission tomography, or PET scans. This imaging technique could pinpoint the buildup of amyloid-beta in the brain. “That’s currently the gold standard for Alzheimer’s diagnosis,” Weiner notes.
NIH-funded research has continued to build on this advance. For example, a recent study found that using PET to image tau in the brain was even better at predicting future brain degeneration than measuring amyloid-beta. PET imaging of tau may also aid future clinical trials.
PET scans, however, are extremely expensive and require exposure to radiation. Other tests have become available that don’t require brain imaging. One type measures amyloid-beta levels in the cerebrospinal fluid, or CSF, instead. Though less expensive than PET scans, these tests require a lumbar puncture—the insertion of a needle into the spinal canal. This procedure must be done by a specialist, typically takes 30 to 60 minutes, and not everyone can have one.
Building on the promise of these methods, researchers have been working to develop quicker and easier tests for biomarkers of Alzheimer’s disease. Blood tests would be ideal. “The advantages of a blood test are that it’s easy to obtain, it’s very fast, and it’s much cheaper,” Masliah says.
Such tests have the potential to greatly widen access to early diagnosis and treatment for Alzheimer’s disease. “Most people with Alzheimer’s disease are cared for by general practitioners, not specialty clinics,” Sperling explains. “If these blood tests are accurate, people who aren’t Alzheimer’s specialists could use them to figure out how best to care for patients.”
Better tests for Alzheimer’s
The most obvious target for an Alzheimer’s blood test is amyloid-beta. Dysfunctional forms of this protein can build up in the brain long before damage to neurons occurs. Studies have found that these abnormal proteins can also make their way into the bloodstream.
An NIH-funded research team recently developed a test that could detect clumps of amyloid-beta in the bloodstream. It could distinguish these clumps from other types of amyloid that aren’t thought to harm the brain. The test proved better at predicting the later development of Alzheimer’s dementia than other more invasive tests.
Another candidate for a blood-based biomarker of Alzheimer’s is tau. Tau tangles in the brain are thought to occur later in Alzheimer’s disease, but blood tests for some forms of tau have shown promise for early detection. One type of tau, called p-tau-217, for example, has recently shown especially strong results.
Two recent NIH-funded studies found that blood tests for p-tau-217 identified, with about 90% accuracy, people with Alzheimer’s-related changes in the brain that were later verified by PET imaging.
Another class of promising biomarkers for Alzheimer’s are molecules that get released when neurons and other types of brain cells are damaged. Some of these molecules aren’t unique to Alzheimer’s and so might also be used for early diagnoses of different types of dementia.
Tests for other dementias
Other types of dementia may be rarer than Alzheimer’s but no less devastating. Potential biomarkers for the early detection of other forms of dementia include a protein called α-synuclein. This may be able to help diagnose Lewy body dementia, as well as Parkinson’s disease, which can also affect the brain.
Researchers have also been studying potential biomarkers for a group of disorders that have some similarities to Alzheimer’s, collectively known as frontotemporal disorders, or FTD. These rose in the public consciousness in 2023, when the family of actor Bruce Willis revealed his diagnosis.
Unlike Alzheimer’s, FTD tends to occur most often in those under the age of 65 and starts with behavior or language changes. But like Alzheimer’s, FTD can be difficult to diagnose before symptoms develop—and sometimes even afterward—especially in people with no family history of the disease.
Recently, NIH-funded researchers showed that blood tests for a protein called neurofilament light chain, or NfL, hold promise for early detection of FTD and related conditions, such as amyotrophic lateral sclerosis, or ALS. NfL is one of the proteins released when nerve cells in the brain or body are damaged.
“NfL won’t ever, on its own, be a diagnostic biomarker for any of the neurodegenerative diseases,” explains Dr. Tania Gendron, an FTD researcher at the Mayo Clinic. “However, there are some diseases, like FTD or ALS, that have symptoms that might be mimicked by other diseases that are not neurodegenerative. So what NfL could do is help facilitate a diagnosis by ruling out or ruling in neuronal injury.”
Gendron and her collaborators found that, among people who carried a genetic risk factor for FTD, NfL levels in the blood were higher in those who later developed symptoms compared with those who didn’t. Higher levels of NfL in blood samples taken at the start of the study were also associated with faster cognitive decline.
Having a test to catch FTD early would enable testing of treatments that aim to halt the disease before it causes substantial damage. But while NfL can detect neural damage, it can’t tell what molecular mechanisms set off that process. Many different genetic factors can affect FTD, and these will likely require different treatment strategies.
“We believe it’s ideal to stop the molecular underpinnings of FTD as early as possible in its tracks,” Gendron explains. “It’s like one domino hits another domino, and then it just becomes chaos, like a snowball going down a mountain. So if you could halt the first key molecular events that trigger the disease, then you’d be better able to, hopefully, prevent worsening of that disease.”
Improving diversity, improving accuracy
Several blood tests for Alzheimer’s disease have already received a type of certification called CLIA, which means the test reliably measures what it claims to measure. To date, none have been approved by the Food and Drug Administration for guiding treatment. But the tests that have reached the CLIA stage are already being adopted by memory clinics and large, ongoing treatment studies, Sperling explains.

For example, Sperling’s team has been running large NIH-funded clinical trials of drugs to prevent the progression of Alzheimer’s disease. As imaging biomarkers have become more widespread, they’ve pushed the timeline for intervention earlier and earlier—from full-blown Alzheimer’s disease to its precursor, called mild cognitive impairment, and finally to people without any symptoms of cognitive decline.
For one of their ongoing studies, called AHEAD, “the first step for screening is now a blood test,” Sperling says. “In terms of therapeutics that target amyloid buildup, treating earlier is better, probably even before symptoms develop. And the blood-based biomarkers are pretty good at finding people at that early stage of amyloid buildup.”
One roadblock has been that such blood tests haven’t been widely tested in diverse populations, Weiner explains. In the past, biomarker tests for dementia have largely enrolled people from white, well-educated, relatively wealthy groups. “But these tests should be validated in populations that really look like America,” Weiner says.
The relationship between amyloid PET levels and dementia outcomes, for example, appears to vary across different populations. “We don’t really understand the relationship of amyloid PET to Alzheimer’s pathology in the Black population very well,” Weiner notes. That could lead to sub-optimal treatment for many patients.
For almost 20 years, Weiner and his team have been running a large NIH-funded study to improve biomarkers for Alzheimer’s disease. In the last year, they’ve doubled participation among people from underrepresented groups.
“Underrepresented means Black, Latino, Asian/Pacific Islander, but also anyone with 12 years of education or less. And people from rural areas are also considered underrepresented,” he adds. His team hopes to eventually have more than half of their participants come from underrepresented groups. Having diversity during a test’s validation greatly increases its likelihood of being useful across populations.
Sperling’s group is also working to diversify their study participants. They noticed that many people from communities of color weren’t meeting the amyloid PET criteria for study participation, regardless of their symptoms. So this year, they started a new study that uses blood tests to explore what happens in the brains of people who have low levels of amyloid on PET scans but are at risk for developing dementia in the future.
The team hopes to find patterns in blood tests that will help inform future prevention strategies. “I think we’re getting closer to precision medicine,” Sperling says. “If amyloid is not the main contributor to Alzheimer’s symptoms in some communities, then we’ll need other drugs.”
Reliable, early biomarkers will also allow for the testing of strategies that may sound like science fiction today, such as anti-Alzheimer’s vaccines, Sperling explains. “That’s the future—that’s the way you’d be able to get preventive treatment to millions, or tens or hundreds of millions of people who need it.”
“What’s happening with biomarkers in Alzheimer’s disease is a real breakthrough; it has completely transformed the field,” Masliah says. “But I can’t stress enough the importance of people participating in these studies. All these breakthroughs have been not only due to the great work that the researchers are doing, but to the people who are volunteering to take part.”
Learn about joining a study of Alzheimer’s disease.
-NIH news










